Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers
MD, MPhil Benjamin S Abella, BA Eliana L Jolkovsky, MPH Barbara T Biney, MD Julie E Uspal, MD, PhD Matthew C Hyman, MD Ian Frank, PhD Scott E Hensley, MD, PhD Saar Gill, MD Dan T Vogl, MSCE Ivan Maillard, MD, PhD Daria V Babushok, MD Alexander C Huang, PhD Sunita D Nasta, MD Jennifer C Walsh, E Paul Wiletyo, PhD; Phyllis A Gimotty, MD Michael C Milone, PhD Ravi K Amaravadi
JAMA Internal Medicine, doi:10.1001/jamainternmed.2020.6319
and the Prevention and Treatment of COVID-19 With Hydroxychloroquine (PATCH) Investigators IMPORTANCE Health care workers (HCWs) caring for patients with coronavirus disease 2019 (COVID-19) are at risk of exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Currently, to our knowledge, there is no effective pharmacologic prophylaxis for individuals at risk. OBJECTIVE To evaluate the efficacy of hydroxychloroquine to prevent transmission of SARS-CoV-2 in hospital-based HCWs with exposure to patients with COVID-19 using a pre-exposure prophylaxis strategy. DESIGN, SETTING, AND PARTICIPANTS This randomized, double-blind, placebo-controlled clinical trial (the Prevention and Treatment of COVID-19 With Hydroxychloroquine Study) was conducted at 2 tertiary urban hospitals, with enrollment from April 9, 2020, to July 14, 2020; follow-up ended August 4, 2020. The trial randomized 132 full-time, hospital-based HCWs (physicians, nurses, certified nursing assistants, emergency technicians, and respiratory therapists), of whom 125 were initially asymptomatic and had negative results for SARS-CoV-2 by nasopharyngeal swab. The trial was terminated early for futility before reaching a planned enrollment of 200 participants. INTERVENTIONS Hydroxychloroquine, 600 mg, daily, or size-matched placebo taken orally for 8 weeks. MAIN OUTCOMES AND MEASURES The primary outcome was the incidence of SARS-CoV-2 infection as determined by a nasopharyngeal swab during the 8 weeks of treatment. Secondary outcomes included adverse effects, treatment discontinuation, presence of SARS-CoV-2 antibodies, frequency of QTc prolongation, and clinical outcomes for SARS-CoV-2-positive participants.
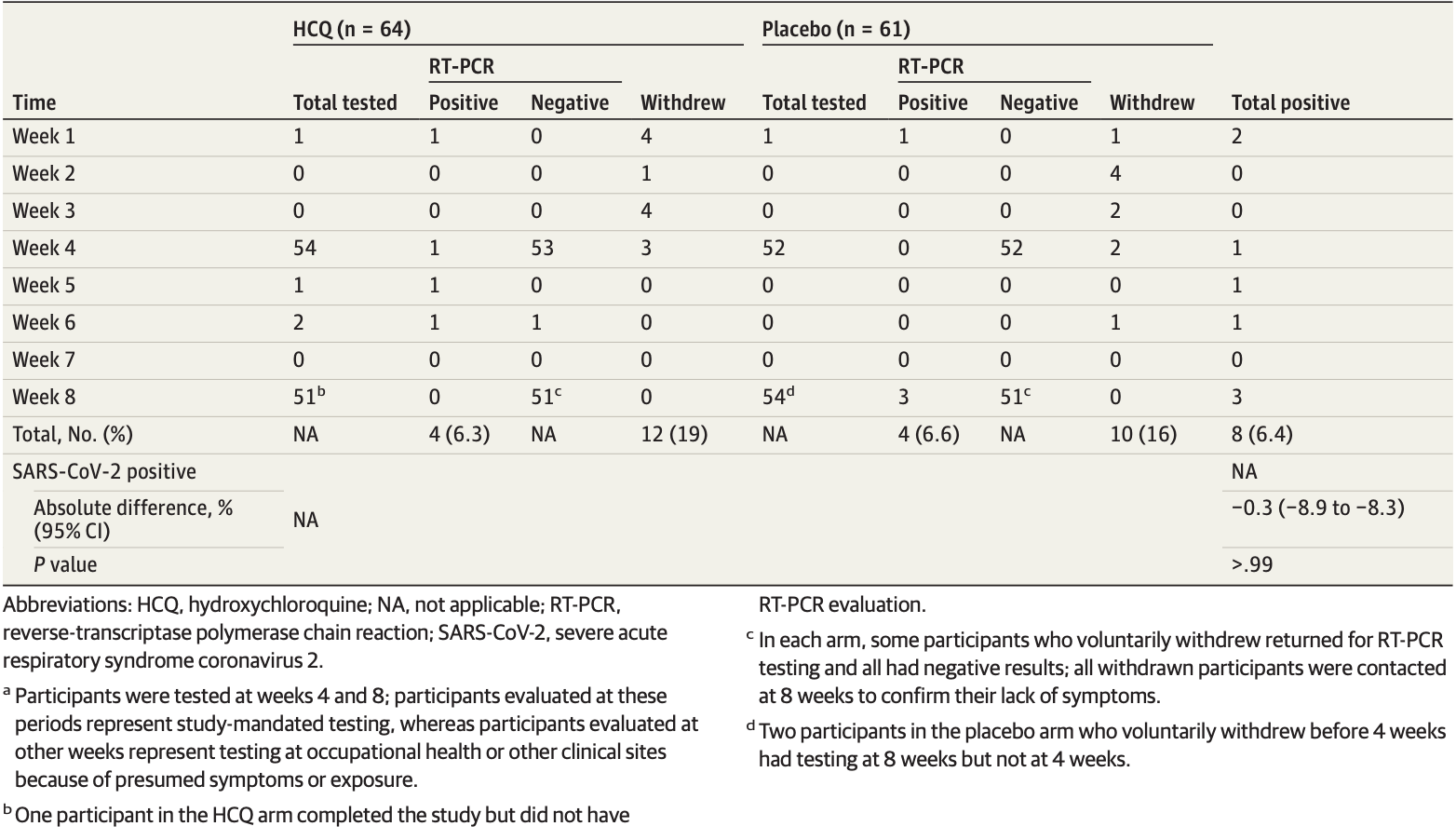
RESULTS Of the 132 randomized participants (median age, 33 years [range, 20-66 years]; 91 women [69%]), 125 (94.7%) were evaluable for the primary outcome. There was no significant difference in infection rates in participants randomized to receive hydroxychloroquine compared with placebo (4 of 64 [6.3%] vs 4 of 61 [6.6%]; P > .99). Mild adverse events were more common in participants taking hydroxychloroquine compared with placebo (45% vs 26%; P = .04); rates of treatment discontinuation were similar in both arms (19% vs 16%; P = .81). The median change in QTc (baseline to 4-week evaluation) did not differ between arms (hydroxychloroquine: 4 milliseconds; 95% CI, −9 to 17; vs placebo: 3 milliseconds; 95% CI, −5 to 11; P = .98). Of the 8 participants with positive results for SARS-CoV-2 (6.4%), 6 developed viral symptoms; none required hospitalization, and all clinically recovered.
CONCLUSIONS AND RELEVANCE In this randomized clinical trial, although limited by early termination, there was no clinical benefit of hydroxychloroquine administered daily for 8 weeks as pre-exposure prophylaxis in hospital-based HCWs exposed to patients with COVID-19.
Serological testing for the presence of anti-spike protein RBD IgM and IgG and nucleocapsid protein IgG (eTable 3 in Supplement 3) demonstrated that only 2 participants had anti-nucleocapsid IgG at baseline. Both participants had a negative SARS-CoV-2 RT-P CR test result, and these participants did not possess anti-spike protein RBD IgG at baseline. At the end of the 8 weeks, there were more positive participants treated with hydroxychloroquine (4 [7.4%]) compared with placebo (2 [3.7%]) who had an IgG antibody against SARS-CoV-2 (P = .40). All participants who developed antibodies also converted to SARS-CoV-2 positive status (eTable 4 in Supplement 3). At least 1 dose of study medication was taken by 65 participants in each arm; therefore, these participants were evaluable for adverse events (Table 3 ). The mean (SD) percentage of total pill counts prescribed that were actually taken during study treatment was 97% (8%) (hydroxychloroquine) and 98% (4%) (placebo). No participants in this study experienced grade 3 or 4 adverse events on the Common Toxicity Criteria for Adverse Events scale, hospitalizations, or death. However, there was a significant increase in any adverse events in the hydroxychloroquine arm vs placebo (45% vs 26%; P = .03), with increased diarrhea in participants receiving hydroxychloroquine compared with placebo (32% vs 12%; P = .01). No cardiac events (eg, syncope and arrhythmias) were observed. There was no significant difference in the median of..
References
Bampoe, Lucas, Neall, A cross-sectional study of immune seroconversion to SARS-CoV-2 in frontline maternity health professionals, Anaesthesia,
doi:10.1111/anae.15229Chu, Akl, Duda, Solo, Yaacoub et al., COVID-19 Systematic Urgent Review Group Effort (SURGE) Study Authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis, Lancet,
doi:10.1016/S0140-6736(20)31142-9Giudicessi, Noseworthy, Friedman, Ackerman, Ml et al., Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19), Mayo Clin Proc,
doi:10.1016/j.mayocp.2020.05.005Grau-Pujol, Camprubí, Marti-Soler, Fernández-Pardos, Guinovart et al., Pre-exposure prophylaxis with hydroxychloroquine for high-risk healthcare workers during the COVID-19 pandemic: a structured summary of a study protocol for a multicentre, double-blind randomized controlled trial, Trials,
doi:10.1186/s13063-020-04621-7Hernandez, Roman, Pasupuleti, Barboza, White, Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review, Ann Intern Med,
doi:10.7326/M20-2496Horby, Lim, Emberson, Dexamethasone in hospitalized patients with Covid-19-preliminary Report, N Engl J Med,
doi:10.1056/NEJMoa2021436Nanni, Viale, Vertogen, PROTECT Trial: a cluster-randomized study with hydroxychloroquine versus observational support for prevention or early-phase treatment of coronavirus disease (COVID-19): a structured summary of a study protocol for a randomized controlled trial, Trials,
doi:10.1186/s13063-020-04527-4Olender, Perez, Go, Remdesivir for severe COVID-19 versus a cohort receiving standard of care, Clin Infect Dis,
doi:10.1093/cid/ciaa1041Shippey, Wagler, Collamer, Juurlink, Boulware et al., Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection, Cleve Clin J Med,
doi:10.1056/NEJMe2020388Tai, Shah, Doubeni, Disproportionate impact of COVID-19 on racial and ethnic minorities in the United States, Clin Infect Dis. Published online,
doi:10.1093/cid/ciaa815Wright, Tan, Walmsley, Protecting frontline health care workers from COVID-19 with hydroxychloroquine pre-exposure prophylaxis: a structured summary of a study protocol for a randomised placebo-controlled multisite trial in Toronto, Canada, Trials,
doi:10.1186/s13063-020-04577-8Ye, Zhang, Zhang, Impact of comorbidities on patients with COVID-19: a large retrospective study in Zhejiang, China, J Med Virol. Published,
doi:10.1002/jmv.26183Zhang, Schwartz, Spatial disparities in coronavirus incidence and mortality in the United States: An ecological analysis as of May
DOI record:
{
"DOI": "10.1001/jamainternmed.2020.6319",
"ISSN": [
"2168-6106"
],
"URL": "http://dx.doi.org/10.1001/jamainternmed.2020.6319",
"author": [
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Abella",
"given": "Benjamin S.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Jolkovsky",
"given": "Eliana L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Biney",
"given": "Barbara T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Uspal",
"given": "Julie E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Cardiology, Department of Medicine University of Pennsylvania, Philadelphia"
}
],
"family": "Hyman",
"given": "Matthew C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine University of Pennsylvania, Philadelphia"
}
],
"family": "Frank",
"given": "Ian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology, University of Pennsylvania, Philadelphia"
}
],
"family": "Hensley",
"given": "Scott E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Abramson Cancer Center and Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Gill",
"given": "Saar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Abramson Cancer Center and Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Vogl",
"given": "Dan T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Abramson Cancer Center and Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Maillard",
"given": "Ivan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Abramson Cancer Center and Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Babushok",
"given": "Daria V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Abramson Cancer Center and Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Huang",
"given": "Alexander C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Abramson Cancer Center and Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Nasta",
"given": "Sunita D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Abramson Cancer Center and Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Walsh",
"given": "Jennifer C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, Epidemiology and Informatics, University of Pennsylvania, Philadelphia"
}
],
"family": "Wiletyo",
"given": "E. Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, Epidemiology and Informatics, University of Pennsylvania, Philadelphia"
}
],
"family": "Gimotty",
"given": "Phyllis A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology, University of Pennsylvania, Philadelphia"
}
],
"family": "Milone",
"given": "Michael C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Abramson Cancer Center and Division of Hematology-Oncology, Department of Medicine, University of Pennsylvania, Philadelphia"
}
],
"family": "Amaravadi",
"given": "Ravi K.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "Prevention and Treatment of COVID-19 With Hydroxychloroquine (PATCH) Investigators",
"sequence": "additional"
}
],
"container-title": "JAMA Internal Medicine",
"container-title-short": "JAMA Intern Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
9,
30
]
],
"date-time": "2020-09-30T21:02:12Z",
"timestamp": 1601499732000
},
"deposited": {
"date-parts": [
[
2021,
2,
2
]
],
"date-time": "2021-02-02T05:41:52Z",
"timestamp": 1612244512000
},
"indexed": {
"date-parts": [
[
2024,
4,
5
]
],
"date-time": "2024-04-05T02:15:41Z",
"timestamp": 1712283341847
},
"is-referenced-by-count": 141,
"issue": "2",
"issued": {
"date-parts": [
[
2021,
2,
1
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2021,
2,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamainternalmedicine/articlepdf/2771265/jamainternal_abella_2020_oi_200089_1611344111.08576.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "195",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2021,
2,
1
]
]
},
"published-print": {
"date-parts": [
[
2021,
2,
1
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"article-title": "Estimation of excess deaths associated with the COVID-19 pandemic in the United States, March to May 2020.",
"author": "Weinberger",
"journal-title": "JAMA Intern Med",
"key": "ioi200089r2",
"volume": "e203391",
"year": "2020"
},
{
"DOI": "10.1111/jrh.v36.3",
"article-title": "Spatial disparities in coronavirus incidence and mortality in the United States: An ecological analysis as of May 2020.",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "433",
"issue": "3",
"journal-title": "J Rural Health",
"key": "ioi200089r3",
"volume": "36",
"year": "2020"
},
{
"article-title": "Disproportionate impact of COVID-19 on racial and ethnic minorities in the United States.",
"author": "Tai",
"journal-title": "Clin Infect Dis",
"key": "ioi200089r4"
},
{
"article-title": "Impact of comorbidities on patients with COVID-19: a large retrospective study in Zhejiang, China.",
"author": "Ye",
"journal-title": "J Med Virol",
"key": "ioi200089r5"
},
{
"DOI": "10.1111/anae.15229",
"article-title": "A cross-sectional study of immune seroconversion to SARS-CoV-2 in frontline maternity health professionals.",
"author": "Bampoe",
"doi-asserted-by": "crossref",
"journal-title": "Anaesthesia",
"key": "ioi200089r6",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31142-9",
"article-title": "Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis.",
"author": "Chu",
"doi-asserted-by": "publisher",
"first-page": "1973",
"issue": "10242",
"journal-title": "Lancet",
"key": "ioi200089r7",
"volume": "395",
"year": "2020"
},
{
"article-title": "Dexamethasone in hospitalized patients with Covid-19—preliminary Report.",
"author": "Horby",
"journal-title": "N Engl J Med",
"key": "ioi200089r8",
"year": "2020"
},
{
"article-title": "Remdesivir for severe COVID-19 versus a cohort receiving standard of care.",
"author": "Olender",
"journal-title": "Clin Infect Dis",
"key": "ioi200089r9",
"year": "2020"
},
{
"DOI": "10.1186/s13063-020-04621-7",
"article-title": "Pre-exposure prophylaxis with hydroxychloroquine for high-risk healthcare workers during the COVID-19 pandemic: a structured summary of a study protocol for a multicentre, double-blind randomized controlled trial.",
"author": "Grau-Pujol",
"doi-asserted-by": "publisher",
"first-page": "688",
"issue": "1",
"journal-title": "Trials",
"key": "ioi200089r10",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.7326/M20-2496",
"article-title": "Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review.",
"author": "Hernandez",
"doi-asserted-by": "publisher",
"first-page": "287",
"issue": "4",
"journal-title": "Ann Intern Med",
"key": "ioi200089r11",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.3949/ccjm.85a.17034",
"article-title": "Hydroxychloroquine: an old drug with new relevance.",
"author": "Shippey",
"doi-asserted-by": "publisher",
"first-page": "459",
"issue": "6",
"journal-title": "Cleve Clin J Med",
"key": "ioi200089r12",
"volume": "85",
"year": "2018"
},
{
"DOI": "10.1503/cmaj.200528",
"article-title": "Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection.",
"author": "Juurlink",
"doi-asserted-by": "publisher",
"first-page": "E450",
"issue": "17",
"journal-title": "CMAJ",
"key": "ioi200089r13",
"volume": "192",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2016638",
"article-title": "A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19.",
"author": "Boulware",
"doi-asserted-by": "publisher",
"first-page": "517",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "ioi200089r14",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMe2020388",
"article-title": "Hydroxychloroquine for the prevention of Covid-19—searching for evidence.",
"author": "Cohen",
"doi-asserted-by": "publisher",
"first-page": "585",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "ioi200089r15",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/j.mayocp.2020.03.024",
"article-title": "Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19).",
"author": "Giudicessi",
"doi-asserted-by": "publisher",
"first-page": "1213",
"issue": "6",
"journal-title": "Mayo Clin Proc",
"key": "ioi200089r16",
"volume": "95",
"year": "2020"
},
{
"article-title": "Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19).",
"author": "Puntmann",
"journal-title": "JAMA Cardiol",
"key": "ioi200089r18",
"volume": "e203557",
"year": "2020"
},
{
"DOI": "10.1016/j.mayocp.2020.05.005",
"article-title": "Acute QT interval modifications during hydroxychloroquine-azithromycin treatment in the context of COVID-19 infection.",
"author": "Voisin",
"doi-asserted-by": "publisher",
"first-page": "1696",
"issue": "8",
"journal-title": "Mayo Clin Proc",
"key": "ioi200089r19",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2558-4",
"article-title": "Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates.",
"author": "Maisonnasse",
"doi-asserted-by": "crossref",
"journal-title": "Nature",
"key": "ioi200089r21",
"year": "2020"
},
{
"DOI": "10.1186/s13063-020-04577-8",
"article-title": "Protecting frontline health care workers from COVID-19 with hydroxychloroquine pre-exposure prophylaxis: a structured summary of a study protocol for a randomised placebo-controlled multisite trial in Toronto, Canada.",
"author": "Wright",
"doi-asserted-by": "publisher",
"first-page": "647",
"issue": "1",
"journal-title": "Trials",
"key": "ioi200089r22",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1186/s13063-020-04527-4",
"article-title": "PROTECT Trial: a cluster-randomized study with hydroxychloroquine versus observational support for prevention or early-phase treatment of coronavirus disease (COVID-19): a structured summary of a study protocol for a randomized controlled trial.",
"author": "Nanni",
"doi-asserted-by": "publisher",
"first-page": "689",
"issue": "1",
"journal-title": "Trials",
"key": "ioi200089r23",
"volume": "21",
"year": "2020"
},
{
"key": "ioi200089r1",
"unstructured": "US Centers for Disease Control and Prevention. CDC COVID data tracker. Accessed September 23, 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html."
},
{
"key": "ioi200089r17",
"unstructured": "National Cancer Institute. Common terminology criteria for adverse events (CTCAE). Accessed August 16, 2020. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm"
},
{
"key": "ioi200089r20",
"unstructured": "Google. Publicly available COVID-19 datasets. Accessed August 16, 2020. https://cloud.google.com/blog/products/data-analytics/publicly-available-covid-19-data-for-analytics"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2771265"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Internal Medicine"
],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": "Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers",
"type": "journal-article",
"volume": "181"
}