Update: we have not received details for treatment delay. An author reports that treatment initiation time was not recorded:
osf.io. Conflicting estimates are provided in a comment of the article and independent analysis, with reports indicating missing data in the dataset. Also see
medrxiv.org (companion PEP trial), and Pullen et al.
ncbi.nlm.nih.gov, which shows shipping delay for these trials of 19 - 68 hours. Only one third of participants completed enrollment weekdays between 8:00am and 4:00pm, with 44% outside of these hours during the week, and 22% during the weekend. With enrollment up to 4 days after symptom onset, this implies delivery 19 - 164 hours after onset (19 hours would require instantaneous enrollment).
~70 to 140 hour (inc. shipping) delayed outpatient treatment with HCQ showing lower hospitalization/death and faster recovery, but not reaching statistical significance. There was one hospitalized control death and one non-hospitalized HCQ death. It is unclear why there was a non-hospitalized death, external factors such as lack of standard care may be involved. Excluding that case results in one control death and zero HCQ deaths. Details for the hospitalizations and deaths such as medication adherence and treatment delay may be informative but are not provided.
The paper states the end point was changed to symptom severity because they would have required 6,000 participants. However, if the same event rates continued, they would hit 95% significance on the reduction in hospitalization after adding less than 500 patients per arm.
Treatment is relatively late, ~70 to 140 hours after symptoms, including the shipping delay. The paper does not mention the shipping delay but partial details are provided in the study protocol. They are not clear but suggest no shipping on the weekends and a possible 12pm cutoff for same day dispensing and mailing. Assuming that enrollments were evenly distributed between 6am and 12am each day, we get an average of approximately 46 hours shipping delay. We have asked for shipping details and will update with more accurate values when available. In any case the treatment delay is relatively long and there is likely little overlap with the more typical delays used such as 0 - 36 hours for oseltamivir.
Research shows the treatment used in the control arm (folic acid in the USA which was most patients) may have significant efficacy for COVID-19
Deschasaux-Tanguy, Farag, so the true effectiveness of HCQ may be higher than observed. Also see
acpjournals.org.
Kaur et al. note that folic acid is predicted to bind to multiple SARS-CoV-2 proteins, folic acid levels are lower in COVID-19 patients with severe disease, folic acid supplementation may help with COVID-19 associated hypertension and hyperhomocystinemia, and differences in a folic acid-related enzyme could impact COVID-19 geographical severity variation.
The paper compares 0 - 36 hour delayed treatment with oseltamivir (influenza) and ~70 to 140 hour delayed treatment with HCQ (COVID-19), noting that oseltamivir seemed more effective. However, a more comparable study is McLean (2015) who showed that 48 - 119 hour delayed treatment with oseltamivir has no effect. This suggests that HCQ is more effective than oseltamivir, and that HCQ may still have significant effect for some amount of delay beyond the delay where oseltamivir is effective.
6 people were included that enrolled with >4d symptoms, although they do not match the study inclusion criteria. This reduces observed effectiveness. The paper says 56% (236) were enrolled within 1 day of symptoms, but results show only 40% for "<1d", 56% is possibly for <48hrs, we have asked for clarification.
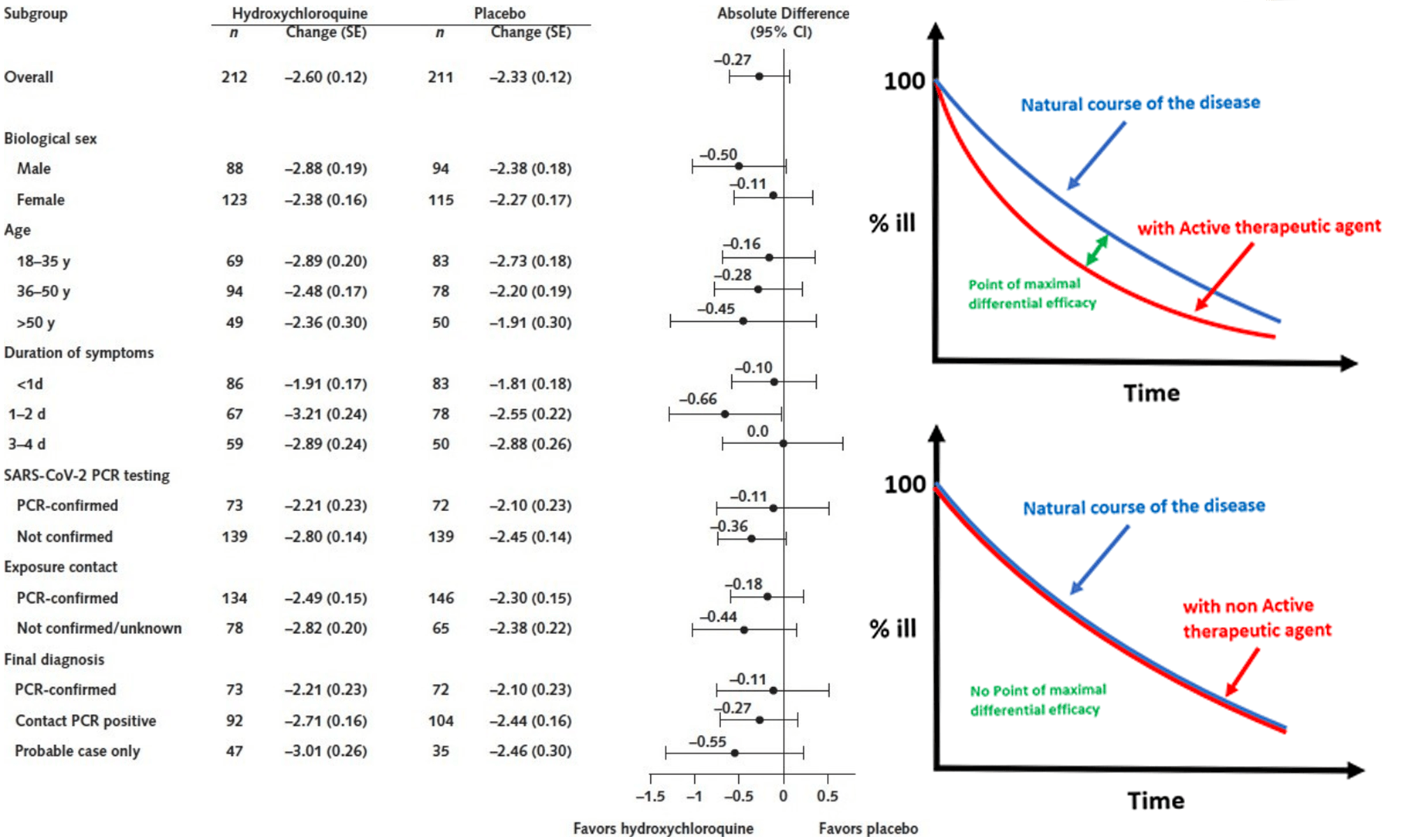
Patients in this study are relatively young and most of them recover without assistance. This reduces the room for a treatment to make improvements. The maximum improvement of an effective treatment would be expected before all patients approach recovery. Authors focus on the end result where most have recovered, but it is more informative to examine the curve and the point of maximum effectiveness. Authors did not collect data for every day but they do have interim results for days 3, 5, 10. The results are consistent with an effective treatment and show a statistically significant improvement, p = 0.05, at day 10 (other unreported days might show increased effectiveness).
Results also show a larger treatment effect for those >50, not statistically significant due to the small sample, but noted as COVID-19 risk dramatically increases with age. The effect may be more visible here because younger patients may on average have more mild cases with less room for improvement. In general patients in this study have relatively mild symptoms on average, limiting the chance to observe improvement.
The study relies on Internet surveys. Known fake surveys were submitted to the similar PEP trial and there could be an unknown number of undetected fake surveys in both trials. The study shows a high incidence of side effects in the placebo arm, which could be in part due to fake entries
.
The granularity change in the histograms of Figure S4 has raised questions . Data on increasing severity, less affected by the lower bound where everyone has recovered, also supports effectiveness .
Treatment delay reporting has changed from the companion PEP trial which reported results for enrollment delays 1, 2, 3, and 4 separately (and from which we can confirm a statistically significant delay-response relationship), while this trial combines 1-2 and 3-4, and adds <1. Since the two trials share reporting (some patients were moved between trials) the reason for the differences are not clear.
RCT of 423 patients with Internet surveys. Medication adherence was only 77% so the true effect of treatment is likely higher. Analysis of primarily low risk patients, authors note the results are not generalizable to the COVID high-risk population. We will update when hearing back on questions asked.
|
risk of death/hospitalization, 36.7% lower, RR 0.63, p = 0.58, treatment 5 of 231 (2.2%), control 8 of 234 (3.4%), NNT 80, COVID-19 adjudicated hospitalization/death.
|
|
risk of hospitalization, 49.4% lower, RR 0.51, p = 0.38, treatment 4 of 231 (1.7%), control 8 of 234 (3.4%), NNT 59, COVID-19 adjudicated hospitalization.
|
|
risk of death/hospitalization, 49.4% lower, RR 0.51, p = 0.29, treatment 5 of 231 (2.2%), control 10 of 234 (4.3%), NNT 47, all hospitalization/death.
|
|
risk of hospitalization, 59.5% lower, RR 0.41, p = 0.17, treatment 4 of 231 (1.7%), control 10 of 234 (4.3%), NNT 39, all hospitalizations.
|
|
risk of no recovery at day 14, 20.0% lower, RR 0.80, p = 0.21, treatment 231, control 234.
|
|
Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates
|
Skipper et al., 16 Jul 2020, Randomized Controlled Trial, USA, peer-reviewed, 24 authors, study period 17 March, 2020 - 20 May, 2020, dosage 800mg once, followed by 600mg in 6 to 8 hours, then 600mg daily for 4 more days, this trial compares with another treatment - results may be better when compared to placebo, trial
NCT04308668 (history).
Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19
MD Caleb P Skipper, BSc Katelyn A Pastick, MS Nicole W Engen, MS Ananta S Bangdiwala, DO, MPH Mahsa Abassi, MD Sarah M Lofgren, MPH Darlisha A Williams, BSc Elizabeth C Okafor, MD Matthew F Pullen, PharmD Melanie R Nicol, PhD Alanna A Nascene, BA Kathy H Hullsiek, PhD Matthew P Cheng, MD Darlette Luke, PharmD Sylvain A Lother, MD Lauren J Mackenzie, MD, MPH Glen Drobot, MD Lauren E Kelly, PhD Ilan S Schwartz, PhD Ryan Zarychanski, MD, MSc Emily G Mcdonald, MD, MSc Todd C Lee, MD, MPH Radha Rajasingham, MD David R Boulware
Annals of Internal Medicine, doi:10.7326/m20-4207
Background: No effective oral therapy exists for early coronavirus disease 2019 . Objective: To investigate whether hydroxychloroquine could reduce COVID-19 severity in adult outpatients. Design: Randomized, double-blind, placebo-controlled trial conducted from 22 March through 20 May 2020. (ClinicalTrials .gov: NCT04308668) Setting: Internet-based trial across the United States and Canada (40 states and 3 provinces). Participants: Symptomatic, nonhospitalized adults with laboratoryconfirmed COVID-19 or probable COVID-19 and high-risk exposure within 4 days of symptom onset. Intervention: Oral hydroxychloroquine (800 mg once, followed by 600 mg in 6 to 8 hours, then 600 mg daily for 4 more days) or masked placebo. Measurements: Symptoms and severity at baseline and then at days 3, 5, 10, and 14 using a 10-point visual analogue scale. The primary end point was change in overall symptom severity over 14 days. Limitation: Only 58% of participants received SARS-CoV-2 testing because of severe U.S. testing shortages.
Conclusion: Hydroxychloroquine did not substantially reduce symptom severity in outpatients with early, mild COVID-19.
References
Beigel, Tomashek, Dodd, ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19 -preliminary report, N Engl J Med,
doi:10.1056/NEJMoa2007764Boulware, Pullen, Bangdiwala, Finding the dose for hydroxychloroquine prophylaxis for COVID-19: the desperate search for effectiveness, Clin Pharmacol Ther,
doi:10.1056/NEJMoa2016638Burke, Midgley, Dratch, Active monitoring of persons exposed to patients with confirmed COVID-19 -United States, January-February 2020,
doi:10.15585/mmwr.mm6909e115Cheng, Papenburg, Desjardins, Diagnostic testing for severe acute respiratory syndrome-related coronavirus 2: a narrative review, Ann Intern Med,
doi:10.7326/M20-1301Geleris, Sun, Platt, Observational study of roquine in hospitalized patients with Covid-19, N Engl J Med,
doi:10.1056/NEJMoa2012410Harris, Taylor, Minor, The REDCap consortium: building an international community of software platform partners, J Biomed Inform,
doi:10.1016/j.jbi.2019.103208Horby, Mafham, Linsell, Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. medRxiv,
doi:10.1101/2020.07.15.2015185219Kaptein, Jacobs, Langendries, Antiviral treatment of SARS-CoV-2-infected hamsters reveals a weak effect of favipiravir and a complete lack of effect for hydroxychloroquine. bioRxiv, mBio,
doi:10.1101/2020.06.19.159053Kucirka, Lauer, Laeyendecker, Variation in falsenegative rate of reverse transcriptase polymerase chain reactionbased SARS-CoV-2 tests by time since exposure, Ann Intern Med,
doi:10.7326/M20-1495Lother, Abassi, Agostinis, Post-exposure prophylaxis or pre-emptive therapy for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): study protocol for a pragmatic randomizedcontrolled trial, Can J Anaesth,
doi:10.1007/s12630-020-01684-7Maisonnasse, Guedj, Contreras, Hydroxychloroquine in the treatment and prophylaxis of SARS-CoV-2 infection in nonhuman primates,
doi:10.21203/rs.3.rs-27223/v1Mcmichael, Currie, Clark, Public Health-Seattle and King County, EvergreenHealth, and CDC COVID-19 Investigation Team. Epidemiology of Covid-19 in a long-term care facility in King County, Washington, N Engl J Med,
doi:10.1056/NEJMoa2005412Million, Lagier, Gautret, Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France, Travel Med Infect Dis,
doi:10.1016/j.tmaid.2020.101738Nicholson, Aoki, Osterhaus, Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group, Lancet
Okafor ; Pullen, Nicol, Hullsiek, Mackenzie, Zarychanski et al., Critical revision of the article for important intellectual content
Pastick, Okafor, Wang, Review: hydroxychloroquine and chloroquine for treatment of SARS-CoV-2 (COVID-19). Open Fo, rum Infect Dis,
doi:10.1093/ofid/ofaa130Ross, Wilson, Papillon-Ferland, COVID-SAFER: deprescribing guidance for hydroxychloroquine drug interactions in olderadults,
doi:10.1111/jgs.1662325Treanor, Hayden, Vrooman, Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group, JAMA
Wang, Zhang, Du, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet,
doi:10.1016/S0140-6736(20)31022-9Yao, Ye, Zhang, In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)